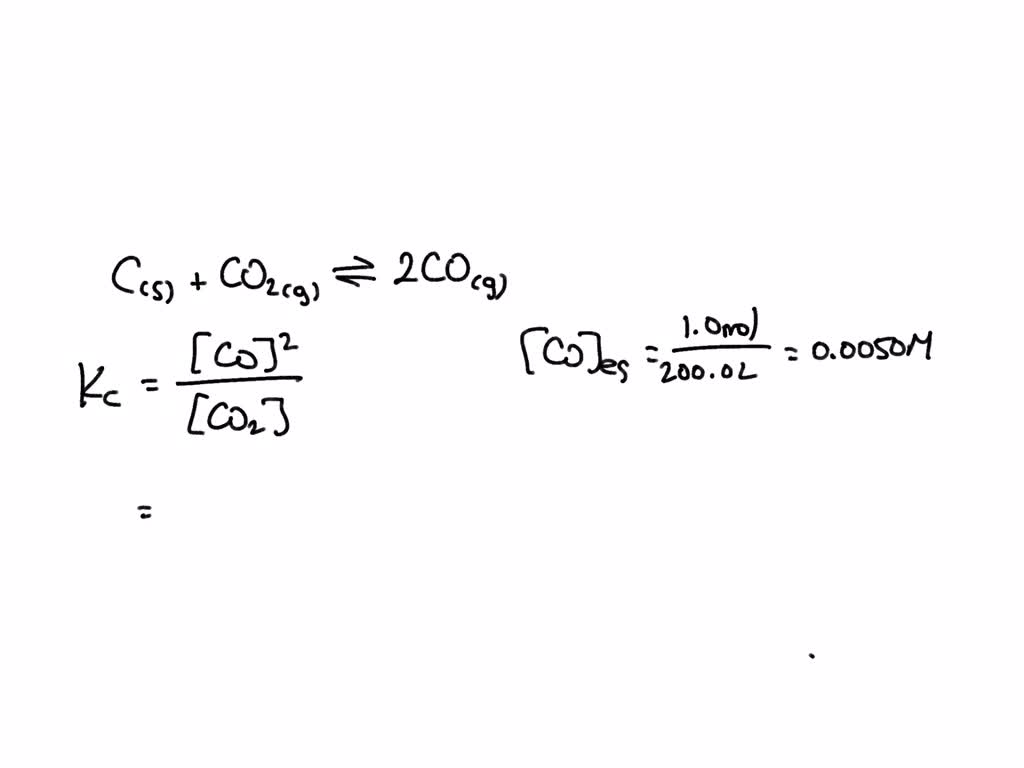
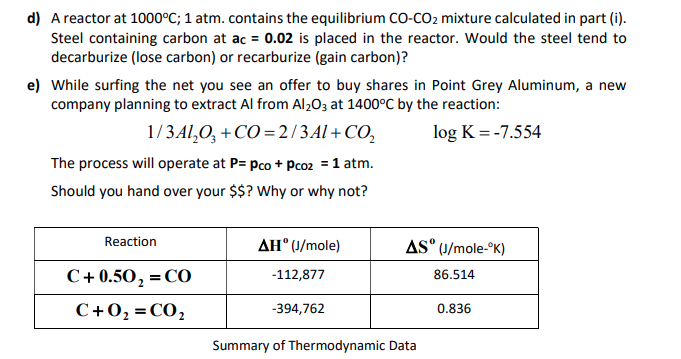
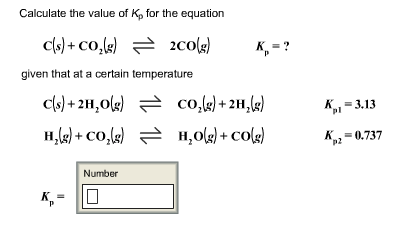
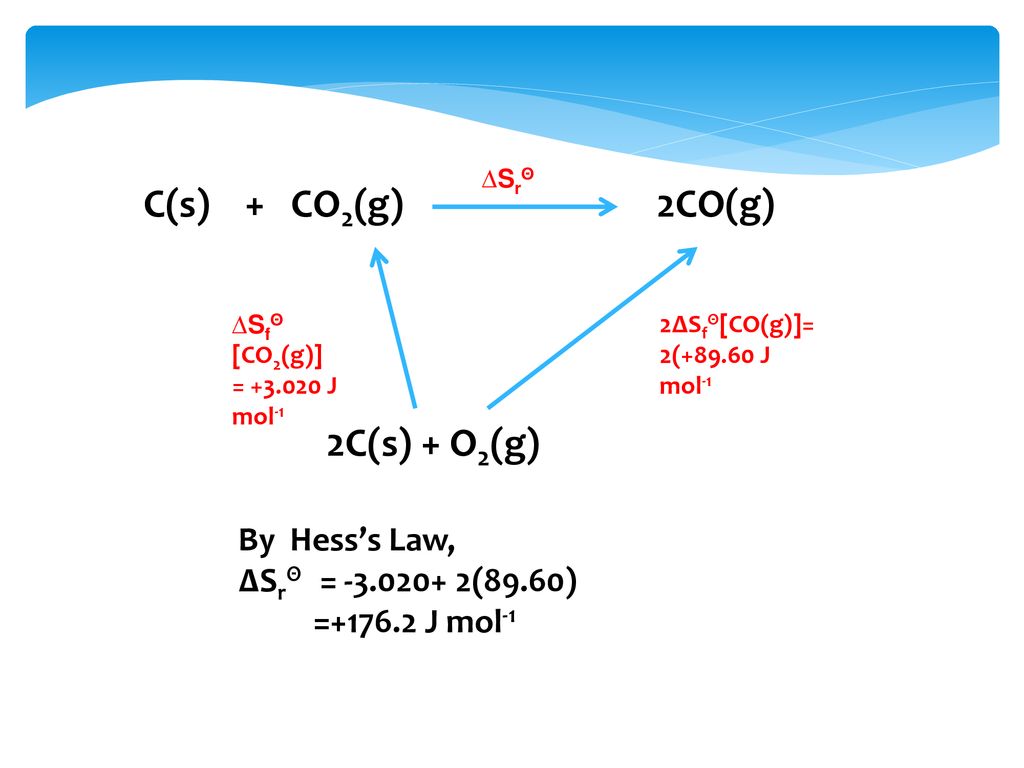
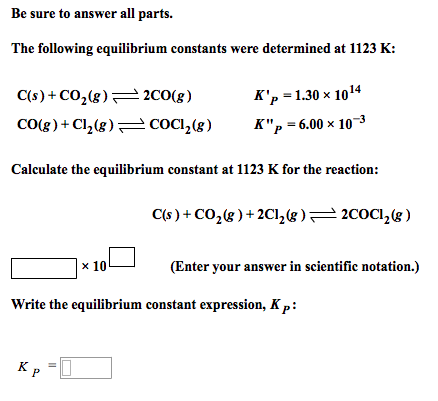
For the reaction, C (s) + CO2 (g) 2CO (g) , the partial pressures of CO2 and CO are 2.0 and 4.0 atm respectively at equilibrium. The Kp for the reaction is:
3.For the equilibrium C(s)+ COg) gives 2CO(g) Kp is 63 atm at 1000 K. If at equilibrium Pco=10Pco2 then total pressure at equilibrium is : 4.A(g) is 90

CH4 and CO2 conversions in the reaction of DRM (CH4 + CO2 → 2H2 + 2CO)... | Download Scientific Diagram

In the reaction C(s) + CO2(g) 2CO(g) the equilibrium pressure is 12 atm. If 50% of CO2 reacts calculate Kp .

For the reaction: C (s)+ CO2 (g) 2CO (g) the partial pressure of CO2 and CO are 2 and 4 atm respectively at equilibrium. Then equilibrium costant for the reaction is -