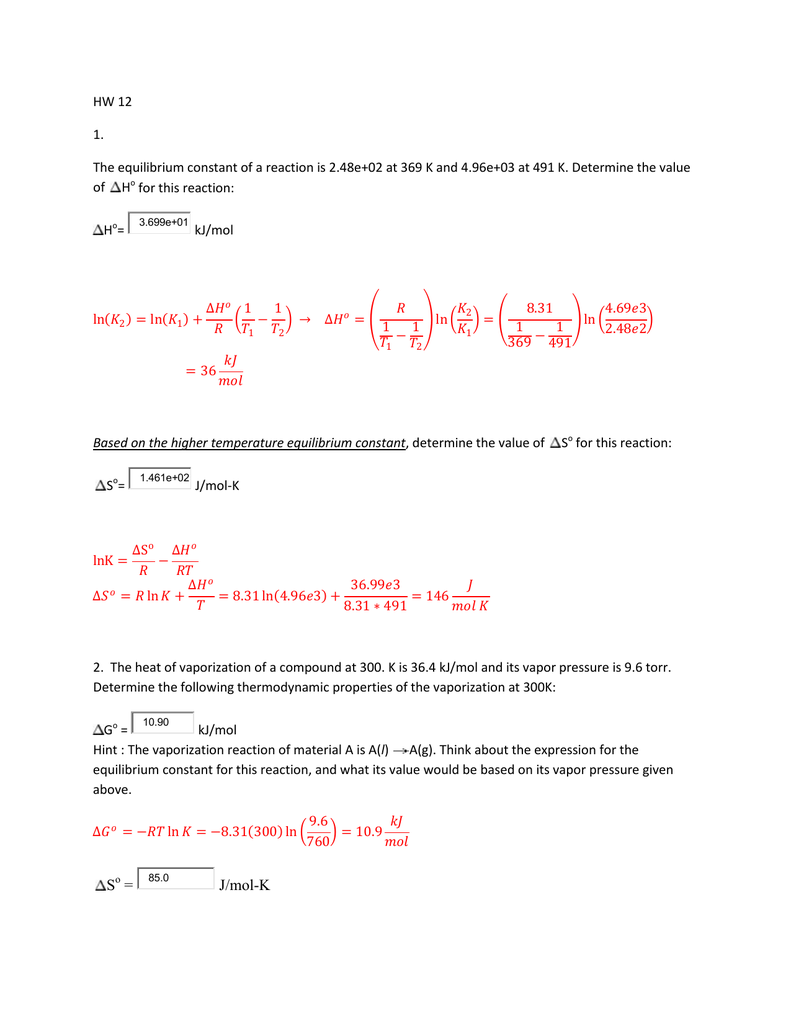
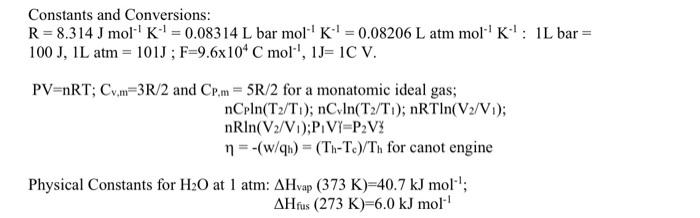
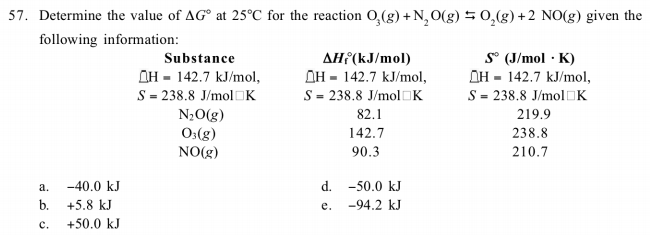
36.) Calculate the rms speed of an ideal diatomic gas having molecular weight 32 gm/mol at Oc If the specific heats at constant pressure and volume are respectively 29.1 J mol1 K

Two moles of helium gas is mixed with three moles of hydrogen molecules (taken to be rigid). What is the molar specific heat of mixture at constant volume ? (R = 8.3

Heat Capacity, CP, (J/mol•K) of Carbon Dioxide as a Function of Temperature and Pressure - Carbon Dioxide Thermodynamic Properties Handbook - Wiley Online Library
Goals of Chapter Assess heat transfer associated with changes in temperature and changes of state. Apply the First Law of Thermodynamics. Define and understand. - ppt download


![ANSWERED] What is the internal energy of 7.00 mol o... - Physical Chemistry - Kunduz ANSWERED] What is the internal energy of 7.00 mol o... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question/raw/52923887-1659262298.2603226.jpeg)