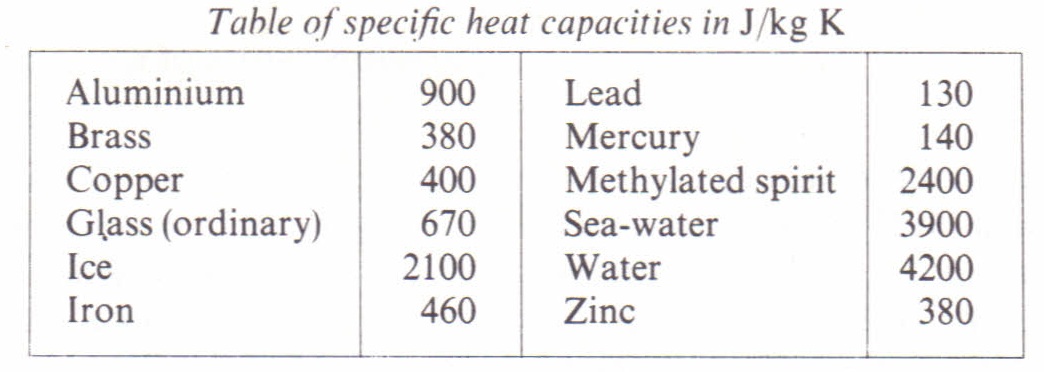
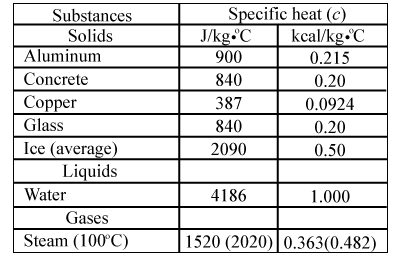
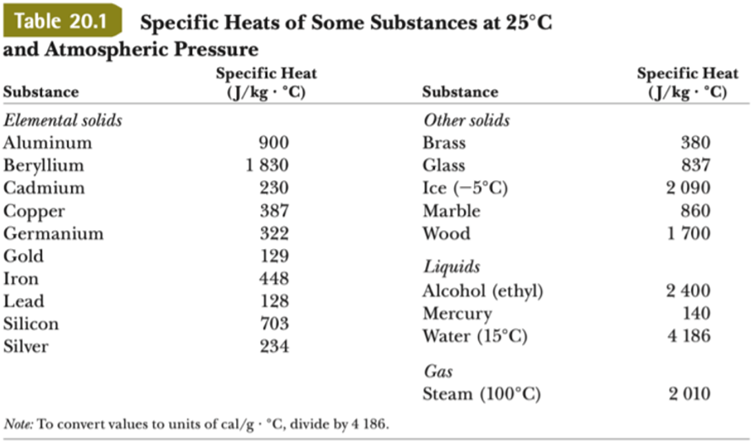
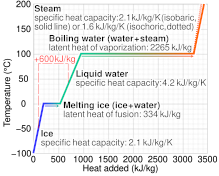
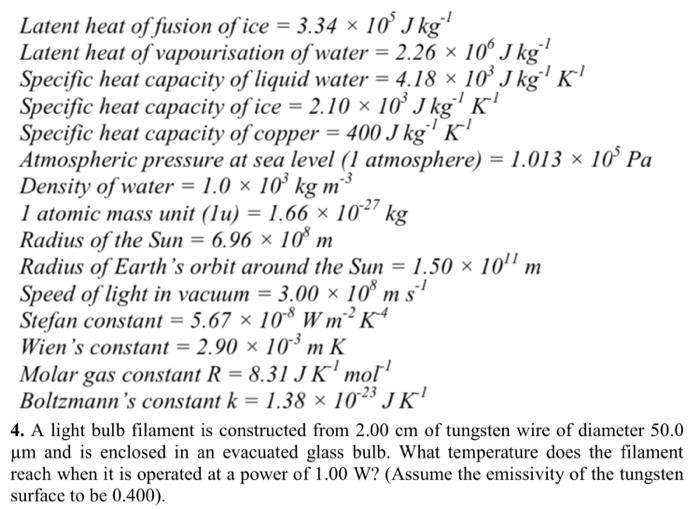
SOLVED: Specific heat capacity of water = 4186 J kg^-1 K^-1 Specific heat capacity of ice = 2090 J kg^-1 K^-1 Latent heat of fusion of ice = 335 kJ kg^-1 Latent

Calculate the heat required to convert 3 kg of ice at - 12^o C kept in a calorimeter to steam at 100^o at atmospheric pressure. (Given: specific heat of ice = 2.100 ×

The temperature of 1.94 kg of water is 34 °C. To cool the water, ice at 0°C is added to it. The desired - brainly.com

The amount of heat energy required to convert 1 kg of ice at - 10^∘C to water at 100^∘C is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat capacity
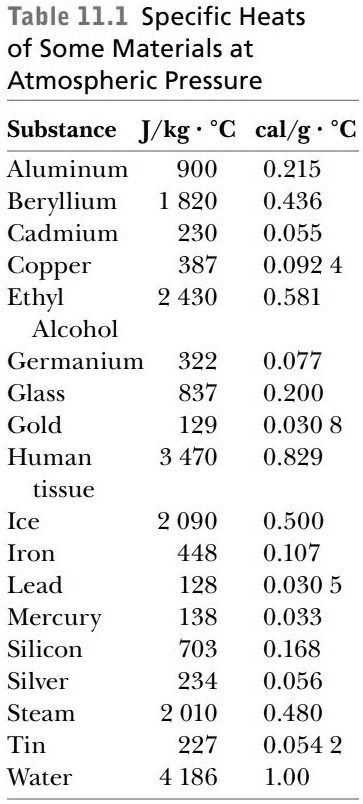
SOLVED: Table 11.1 Specific Heats of Some Materials at Atmospheric Pressure Substance J/kg 'C cal/g Aluminum 900 0.215 Beryllium 820 0.436 Cadmium 230 0.055 Copper 387 0.092 4 Ethyl 2 430 0.581
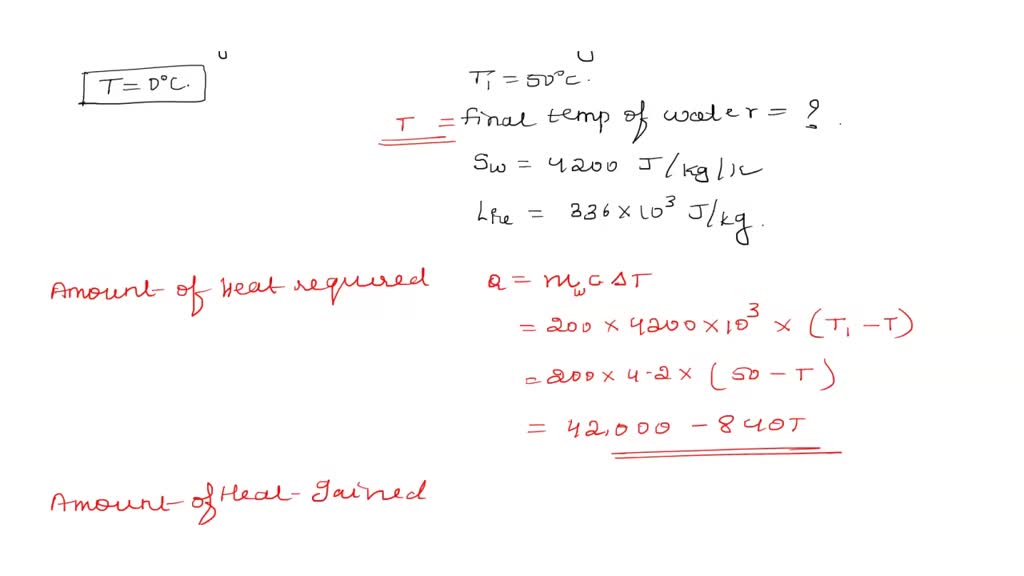
SOLVED: A piece of ice of mass 40 g is added to 200 g of water at 50oC. Calculate the final temperature of water when all the ice has melted. Specific heat

When you heat a substance, you are transferring energy into it by placing it in contact with surroundings that have a higher temperature. - ppt download

The amount of heat energy required to convert 1 kg of ice at - 10^∘C to water at 100^∘C is 7,77,000 J. Calculate the specific latent heat of ice. Specific heat capacity